A) CCl4 only
B) Cl2 only
C) HCl and KCl
D) Cl2 and KCl
E) CCl4 and HCl
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use VSEPR theory to predict the electron-pair geometry and the molecular geometry of nitrogen trichloride, NCl3.
A) The electron-pair geometry is linear, the molecular geometry is linear.
B) The electron-pair geometry is trigonal-planar, the molecular geometry is trigonal-planar.
C) The electron-pair geometry is trigonal-planar, the molecular geometry is bent.
D) The electron-pair geometry is tetrahedral, the molecular geometry is tetrahedral.
E) The electron-pair geometry is tetrahedral, the molecular geometry is trigonal-pyramidal.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a correct Lewis structure for H2SO4?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the following data, what is the F-F bond energy?
A) 500 kJ/mol
B) 150 kJ/mol
C) -150 kJ/mol
D) -695 kJ/mol
E) 695 kJ/mol
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the bond angle in a trigonal planar molecule or ion?
A) 109°
B) 180°
C) 90°
D) 72°
E) 120°
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
The molecular geometry of a molecule whose central atom has four single bonds and one lone pair of electrons is ________.
Correct Answer

verified
trigonal-b...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the total number of electrons (both lone and bond pairs) in the Lewis structure of SO42-?
A) 18
B) 20
C) 32
D) 24
E) 22
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many lone pairs of electrons are assigned to the carbon atom in CO?
A) 0
B) 1
C) 2
D) 3
E) 4
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following pairs of compounds contains ionic bonds?
A) KI and O3
B) NaF and H2O
C) PCl5 and HF
D) Na2SO3 and BH3
E) RbBr and MgS
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atoms having equal or nearly equal electronegativities are expected to form
A) no bonds
B) polar covalent bonds
C) nonpolar covalent bonds
D) ionic bonds
E) covalent bonds
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which combination of atoms is most likely to produce a compound with covalent bonds?
A) K and Br
B) Al and S
C) S and Cl
D) Sn and F
E) Li and I
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many hydrogen atoms are needed to complete the following hydrocarbon structure?
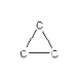
A) 10
B) 12
C) 8
D) 14
E) 6
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct Lewis structure of IF3?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds would be the most polar?
A) N--C
B) N--Si
C) N--P
D) N--Al
E) N--Ga
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the following data reactions: Calculate the energy of an H-Cl bond.
A) 92 kJ/mol
B) 855 kJ/mol
C) 487 kJ/mol
D) 428 kJ/mol
E) 244 kJ/mol
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use VSEPR theory to predict the molecular geometry around either carbon atom in acetylene, C2H2.
A) linear
B) bent
C) trigonal-planar
D) tetrahedral
E) octahedral
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When you draw the Lewis structure for PF2Cl3, how many single bonds, double bonds, and lone pair electrons reside on the central phosphorus atom?
A) 5 single bonds, 0 double bonds, 0 lone pairs
B) 4 single bonds, 1 double bond, 0 lone pairs
C) 5 single bonds, 0 double bonds, 1 lone pair
D) 4 single bonds, 1 double bond, 1 lone pair
E) 3 single bonds, 2 double bonds, 0 lone pairs
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species has a Lewis structure similar to iodine tetrafluoride, IF4-?
A) XeF4
B) SO42-
C) PF4+
D) SF4
E) IO4-
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis structure of which of the following molecules violates the octet rule?
A) SF4
B) NF3
C) OF2
D) HF
E) SiF4
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
In benzene, C6H6, the six carbon atoms are arranged in a ring. Two equivalent Lewis structures can be drawn for benzene. In both structures, the carbon atoms have a trigonal planar geometry. These two equivalent structures are referred to as ________ structures.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 93
Related Exams