A) 1
B) 2
C) 3
D) 4
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an ionic bond?
A) Br-Br
B) C-Cl
C) C-S
D) Na-O
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a secondary (2 ) amine? 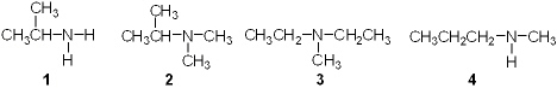
A) 1
B) 2
C) 3
D) 4
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the C=O bond of acetone, (CH3) 2C=O?
A) C 2sp3 + O 2sp2
B) C 2sp2 + O 2p
C) C 2sp2 + O 2sp2
D) C 2sp3 + O 2sp
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is a ketone?
A) CH3CH2COOH
B) CH3CH2CHO
C) CH3CH2CH2OH
D) CH3COCH3
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a tertiary alcohol?
A) CH3CH2OCH3
B) (CH3) 3COH
C) (CH3) 2CHOH
D) CH3CH2CH2OH
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species possesses a formal charge?
A) CCl4
B) SiCl4
C) AlCl4
D) PCl3
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a polar covalent bond?
A) Na-Cl
B) C-Cl
C) C-H
D) Cl-Cl
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electronic configuration of a sodium cation (sodium: atomic number 11) ?
A) 1s22s22p63s1
B) 1s22s22p53s1
C) 1s22s22p6
D) 1s22s22p63s2
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate value of the H-C-H bond angles in a methyl cation, CH3+?
A) 90
B) 109
C) 120
D) 180
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has the highest electronegativity?
A) N
B) C
C) O
D) S
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true regarding resonance structures?
A) Each resonance structure is in rapid equilibrium with all of the other structures
B) The resonance structures may have different energies
C) All resonance structures must have the same arrangement of atoms
D) All resonance structures must have the same number of electrons
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atomic orbitals overlap to form the carbon-hydrogen s bonding molecular orbitals of ethyne, HCºCH?
A) C2p + H1s
B) C2sp + H1s
C) C2sp2 + H1s
D) C2sp3 + H1s
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best represents an sp3 hybridized atomic orbital containing the lone pair of electrons of ammonia, NH3? 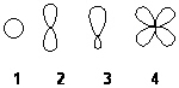
A) 1
B) 2
C) 3
D) 4
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best represents the shape of the 2s atomic orbital of carbon? 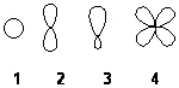
A) 1
B) 2
C) 3
D) 4
F) All of the above
Correct Answer

verified
Correct Answer
verified
Showing 81 - 95 of 95
Related Exams