A) will form hydrogen bonds with water molecules.
B) has a tetrahedral configuration of hybrid electron orbitals for the carbon atom.
C) consists of largely nonpolar covalent bonds.
D) is held together by hydrogen bonds.
E) has a tetrahedral shape and will form hydrogen bonds with water molecules.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the difference between covalent bonds and ionic bonds?
A) Covalent bonds are formed between atoms to form molecules; ionic bonds are formed between atoms to form compounds.
B) Covalent bonds involve the sharing of pairs of electrons between atoms; ionic bonds involve the sharing of single electrons between atoms.
C) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between atoms.
D) Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the sharing of protons between atoms.
E) Covalent bonds involve the transfer of electrons between atoms; ionic bonds involve the sharing of electrons between atoms.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The atomic number of nitrogen is 7. Nitrogen-15 is heavier than nitrogen-14 because the atomic nucleus of nitrogen-15 contains how many neutrons?
A) 6
B) 7
C) 8
D) 12
E) 14
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One difference between carbon-12 ( 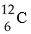 ) and carbon-14 (
) and carbon-14 ( 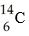 ) is that carbon-14 has
) is that carbon-14 has
A) two more protons than carbon-12.
B) two more electrons than carbon-12.
C) two more neutrons than carbon-12.
D) two more protons and two more neutrons than carbon-12.
E) two more electrons and two more neutrons than carbon-12.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following information to answer the questions below. You are investigating how chemical reactions occur. You place two reactants together and measure the concentration of product at regular intervals. After a time, the amount of product becomes stable. -If you add more product to the solution, you would expect to see
A) a precipitation of the product.
B) an increase in pH.
C) the reactant concentration to remain the same.
D) the reactant concentration to decrease.
E) the reactant concentration to increase.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true for this reaction? 3 H₂ + N₂ ↔ 2 NH₃
A) The reaction is nonreversible.
B) Hydrogen and nitrogen are the reactants of the reverse reaction.
C) Hydrogen and nitrogen are the products of the forward reaction.
D) Ammonia is being formed and decomposed.
E) Hydrogen and nitrogen are being decomposed.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
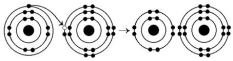 -What is the atomic number of the cation formed in the reaction illustrated above?
-What is the atomic number of the cation formed in the reaction illustrated above?
A) 1
B) 8
C) 10
D) 11
E) 16
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not considered to be a weak molecular interaction?
A) a covalent bond
B) a van der Waals interaction
C) an ionic bond in the presence of water
D) a hydrogen bond
E) both a hydrogen bond and a covalent bond
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following explains most specifically the attraction of water molecules to one another?
A) nonpolar covalent bond
B) polar covalent bond
C) ionic bond
D) hydrogen bond
E) hydrophobic interaction
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
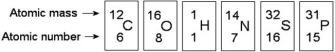 -Based on electron configuration, which of these elements in the figure above would exhibit a chemical behaviour most like that of oxygen?
-Based on electron configuration, which of these elements in the figure above would exhibit a chemical behaviour most like that of oxygen?
A) carbon
B) hydrogen
C) nitrogen
D) sulphur
E) phosphorus
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
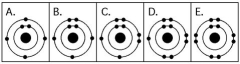 -Which drawing in the figure above depicts the electron configuration of an atom that can form covalent bonds with two hydrogen atoms?
-Which drawing in the figure above depicts the electron configuration of an atom that can form covalent bonds with two hydrogen atoms?
A) A
B) B
C) C
D) D
E) E
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxygen has an atomic number of 8 and a mass number of 16. Thus, what is the atomic mass of an oxygen atom?
A) exactly 8 grams
B) exactly 8 daltons
C) approximately 16 grams
D) approximately 16 daltons
E) 24 amu (atomic mass units)
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Knowing just the atomic mass of an element allows inferences about which of the following?
A) the chemical properties of the element
B) the number of protons in the element
C) the number of neutrons in the element
D) the number of protons plus neutrons in the element
E) both the number of protons and the chemical properties of the element
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following figure to answer the questions below.
 -Which drawing in the figure above is of the electron configuration of a sodium 11Na⁺ ion?
-Which drawing in the figure above is of the electron configuration of a sodium 11Na⁺ ion?
A) A
B) B
C) C
D) D
E) E
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The three types of subatomic particles pertinent to the study of biology are
A) electrons, protons, and neutrinos.
B) electrons, positrons, and neutrons.
C) electrons, protons, and neutrons.
D) electrons, photons, and neutrons.
E) quarks, photons, and gravitons.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of covalent bonds an element with atomic number 8 can make with hydrogen?
A) 1
B) 2
C) 3
D) 4
E) 6
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What factors are most important in determining which elements are most common in living matter?
A) the relative abundances of the elements in Earth's crust and atmosphere
B) the emergent properties of the simple compounds made from these elements
C) the reactivity of the elements with water
D) the chemical stability of the elements
E) both the relative abundances of the elements and the emergent properties of the compounds made from these elements
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Two atoms appear to have the same mass number. These atoms
A) must have the same atomic number.
B) must have the same number of electrons.
C) must have the same chemical properties.
D) must have the same number of protons + neutrons.
E) must have the same atomic number, the same number of protons + neutrons, the same number of electrons, and the same chemical properties.
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following information to answer the questions below. You are investigating how chemical reactions occur. You place two reactants together and measure the concentration of product at regular intervals. After a time, the amount of product becomes stable. -The atomic number of sulphur is 16. Sulphur combines with hydrogen by covalent bonding to form a compound, hydrogen sulfide. Based on the number of valence electrons in a sulphur atom, predict the molecular formula of the compound.
A) HS
B) HS₂
C) H₂S
D) H₃S₂
E) H₄S
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Fluorine has an atomic number of 9 and a mass number of 19. How many electrons are needed to complete the valence shell of a fluorine atom?
A) 1
B) 3
C) 0
D) 7
E) 9
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 90
Related Exams