A) 8
B) 11
C) 15
D) none of these
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 100.mL of 0.400 M Na2SO4 is added to 200.mL of 0.600 M NaCl,what is the concentration of Na+ ions in the final solution? Assume that the volumes are additive.
A) 0) 534 M
B) 0) 667 M
C) 1) 00 M
D) 1) 40 M
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Oxygen can be produced from the catalytic decomposition of KClO3 as shown in the balanced equation below. 2 KClO3 → 2 KCl + 3 O2 What is the percent yield if 3.20 grams of oxygen are formed from the reaction of 12.3 grams of KClO3?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the empirical formula for ethyl fluoride if the compound contains 49.97% carbon,10.51% hydrogen,and 39.52% fluorine by mass?
A) C2H5F
B) C4H10F2
C) C4H10F4
D) C25F2
F) None of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
A balanced equation has the same numbers and kinds of ________ on both sides of the reaction arrow.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the reaction between glucose and oxygen,10.0 g of glucose reacts and 7.50 L of carbon dioxide is formed.What is the percent yield if the density of CO2 is 1.26 g/L? C6H12O6(s) + 6 O2(g) → 6 CO2(g) + 6 H2O(l)
A) 26.1%
B) 40.6%
C) 43.1%
D) 64.5%
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Balance the chemical equation given below,and determine the number of moles of iodine that reacts with 10.0 g of aluminum. _____ Al(s) + _____ I2(s) → _____ Al2I6(s)
A) 0) 247 mol
B) 0) 556 mol
C) 0) 741 mol
D) 1) 11 mol
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Balance the chemical equation given below,and calculate the volume of nitrogen monoxide gas produced when 8.00 g of ammonia is reacted with 12.0 g of oxygen at 25°C? The density of nitrogen monoxide at 25°C is 1.23 g/L. _____ NH3(g) + _____ O2(g) → _____ NO(g) + _____ H2O(l)
A) 7) 32 L
B) 11.1 L
C) 11.5 L
D) 18.8 L
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following diagram represents the reaction of A2 (unshaded spheres) with B (shaded spheres) .What is the balanced chemical equation for this reaction,and what is the limiting reactant? 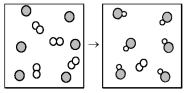
A) A2 + 2B → 2AB;A2 is the limiting reactant.
B) A2 + 2B → 2AB;B is the limiting reactant.
C) 4A2 + 6B → 6AB;A2 is the limiting reactant.
D) 4A2 + 6B → 6AB;B is the limiting reactant.
F) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of aspartic acid,C4O4H7N?
A) 43 g/mol
B) 70 g/mol
C) 133 g/mol
D) 197 g/mol
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which conducts electricity?
A) A large collection of iron atoms
B) A single iron atom
C) Both a large collection of iron atoms and a single iron atom
D) Neither a large collection of iron atoms nor a single iron atom
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many milliliters of 0.550 M hydriodic acid are needed to react with 15.00 mL of 0.217 M CsOH? HI(aq) + CsOH(aq) → CsI(aq) + H2O(l)
A) 0) 0263 mL
B) 0) 169 mL
C) 5) 92 mL
D) 38.0 mL
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration of NO3- ions in a solution prepared by dissolving 15.0 g of Ca(NO3) 2 in enough water to produce 300.mL of solution?
A) 0) 152 M
B) 0) 305 M
C) 0) 403 M
D) 0) 609 M
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many milliliters of a 9.0 M H2SO4 solution are needed to make 0.25 L of a 3.5 M H2SO4 solution?
A) 0) 097 mL
B) 0) 64 mL
C) 97 mL
D) 640 mL
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many milliliters of 0.260 M Na2S are needed to react with 40.00 mL of 0.315 M AgNO3? Na2S(aq) + 2 AgNO3(aq) → 2 NaNO3(aq) + Ag2S(s)
A) 24.2 mL
B) 48.5 mL
C) 66.0 mL
D) 96.9 mL
F) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of CaCl2 are formed when 15.00 mL of 0.00237 M Ca(OH) 2 reacts with excess Cl2 gas? 2 Ca(OH) 2(aq) + 2 Cl2(g) → Ca(OCl) 2(aq) + CaCl2(s) + 2 H2O(l)
A) 0) 00 197 g
B) 0) 00 394 g
C) 0) 0 0789 g
D) 0) 0 507 g
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assume that the unshaded spheres in the buret represent H+ ions,the shaded spheres in the flask represent OH- ions,and you are carrying out a titration of the base with the acid. 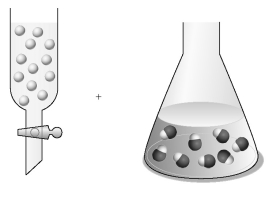 -If the volumes in the buret and the flask are identical and the concentration of the acid in the buret is 0.250 M,what is the concentration of the base in the flask?
-If the volumes in the buret and the flask are identical and the concentration of the acid in the buret is 0.250 M,what is the concentration of the base in the flask?
A) 0) 167 M
B) 0) 250 M
C) 0) 375 M
D) 0) 667 M
F) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
10 g of nitrogen is reacted with 5.0 g of hydrogen to produce ammonia according to the chemical equation shown below.Which one of the following statements is false? N2(g) + 3 H2(g) → 2 NH3(g)
A) 2.8 grams of hydrogen are left over.
B) Hydrogen is the excess reactant.
C) Nitrogen is the limiting reactant.
D) The theoretical yield of ammonia is 15 g.
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the percent yield for the following reaction is 75.0%,and 45.0 g of NO2 are consumed in the reaction,how many grams of nitric acid,HNO3(aq) ,are produced? 3 NO2(g) + H2O(l) → 2 HNO3(aq) + NO(g)
A) 30.8 g
B) 41.1 g
C) 54.8 g
D) 69.3 g
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many cations are in 10.0 g of sodium phosphate?
A) 3) 67 × 1022 cations
B) 1) 10 × 1023 cations
C) 9) 87 × 1024 cations
D) 2) 96 × 1025 cations
F) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 208
Related Exams