A) three ,zero
B) two ,one
C) two ,two
D) one ,two
E) one ,one
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s) .For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules.  -Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will have the bond order?
-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will have the bond order?
A) B2+
B) H2
C) F2+
D) N2+
E) O22-
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s) .For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules.  -Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will be paramagnetic?
-Refer to Diagram 9-1.According to molecular orbital theory,which of the following species will be paramagnetic?
A) O22-
B) Li2
C) O2-
D) C2
E) F2
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning valence bond (VB) theory is/are CORRECT? 1) VB theory can describe molecular bonding in excited states. 2) VB theory assumes that electrons are localized between pairs of atoms. 3) VB theory predicts localized lone pairs of electrons.
A) 1 only
B) 2 only
C) 3 only
D) 2 and 3
E) 1,2,and 3
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s) .For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules.  -Refer to diagram 9-1.Identify the molecule with the shortest bond length.
-Refer to diagram 9-1.Identify the molecule with the shortest bond length.
A) O2
B) C2
C) B2
D) F2
E) N2
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atomic orbitals combine most effectively to form molecular orbitals when
A) electrons in the orbitals have no spins.
B) electrons in the orbitals have the same spin.
C) the atoms have an equal number of valence electrons.
D) the atomic orbitals have similar energies.
E) only d-orbitals are used in bonding.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many sigma ( ) bonds and pi ( ) bonds are in the following molecule? 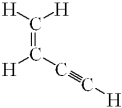
A) seven and three
B) seven and two
C) five and five
D) five and three
E) five and two
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s) .For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. ![Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s) .For oxygen and fluorine,the \sigma <sub>2p</sub> orbital should be lower in energy than the \pi <sub>2p</sub> orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. -Refer to Diagram 9-1.What is the molecular orbital configuration of N<sub>2</sub><sup>2+</sup>? A) [core electrons] ( \sigma <sub>2s</sub>) <sup>2</sup> ( \sigma *<sub>2s</sub>) <sup>2</sup> ( \pi <sub>2p</sub>) <sup>4</sup> ( \sigma <sub>2p</sub>) <sup>2</sup> ( \pi *<sub>2p</sub>) <sup>2</sup> B) [core electrons] ( \sigma <sub>2s</sub>) <sup>2</sup> ( \sigma *<sub>2s</sub>) <sup>2</sup> ( \pi <sub>2p</sub>) <sup>4</sup> C) [core electrons] ( \sigma <sub>2s</sub>) <sup>2</sup> ( \sigma *<sub>2s</sub>) <sup>2</sup> ( \pi <sub>2p</sub>) <sup>2</sup> ( \sigma sub>2p</sub>) <sup>2</sup> D) [core electrons] ( \sigma <sub>2s</sub>) <sup>4</sup> ( \sigma *<sub>2s</sub>) <sup>4</sup> E) [core electrons] ( \sigma <sub>2s</sub>) <sup>2</sup> ( \sigma *<sub>2s</sub>) <sup>2</sup> ( \pi <sub>2p</sub>) <sup>4</sup> ( \sigma <sub>2p</sub>) <sup>2</sup> ( \pi *<sub>2p</sub>) <sup>4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab6c_c408_a16d_5b406ef1d09a_TB4499_00.jpg) -Refer to Diagram 9-1.What is the molecular orbital configuration of N22+?
-Refer to Diagram 9-1.What is the molecular orbital configuration of N22+?
A) [core electrons] ( 2s) 2 ( *2s) 2 ( 2p) 4 ( 2p) 2 ( *2p) 2
B) [core electrons] ( 2s) 2 ( *2s) 2 ( 2p) 4
C) [core electrons] ( 2s) 2 ( *2s) 2 ( 2p) 2 ( sub>2p) 2
D) [core electrons] ( 2s) 4 ( *2s) 4
E) [core electrons] ( 2s) 2 ( *2s) 2 ( 2p) 4 ( 2p) 2 ( *2p) 4
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Benzene,C6H6,consists of a six member ring of sp2 hybridized carbon atoms.Each carbon atom has one unhybridized p orbital.How many 2p bonding,antibonding,and nonbonding molecular orbitals exist for benzene?
A) Three 2p molecular orbitals exist; two bonding and one antibonding.
B) Three 2p molecular orbitals exist; one bonding,one antibonding,and one nonbonding.
C) Six 2p molecular orbitals exist; three bonding and three antibonding.
D) Six 2p molecular orbitals exist; two bonding,two nonbonding,and two antibonding.
E) Twelve 2p molecular orbitals exist; six bonding and six antibonding.
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry around a central atom that is sp2 hybridized,has three sigma bonds,and one pi bond?
A) trigonal-planar
B) trigonal-pyramidal
C) bent
D) T-shaped
E) tetrahedral
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybridization of the nitrogen atom in NH4+ is _____.
A) sp
B) sp2
C) sp3
D) sp4
E) sp6
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many sigma and pi bonds are in the molecule pictured below? 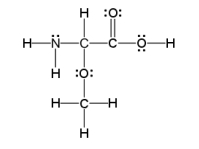
A) thirteen sigma bonds and one pi bond
B) eleven sigma bonds and two pi bonds
C) thirteen sigma bonds and two pi bonds
D) eleven sigma bonds and five pi bonds
E) five sigma bonds and eleven pi bonds
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule will have the following valence molecular orbital level energy diagram?
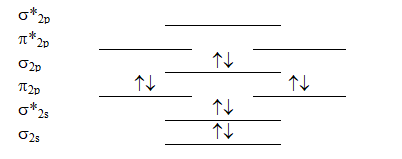
A) Li2
B) Be2
C) B2
D) C2
E) N2
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning molecular orbital (MO) bond theory is/are CORRECT? 1) MO theory can describe molecular bonding in excited states. 2) Molecular orbitals are obtained from the combination of atomic orbitals. 3) MO theory predicts that electrons are delocalized over the molecule through molecular orbitals.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 1,2,and 3
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Upon combustion,ethene (C2H4) is converted to carbon dioxide and water.What change in the hybridization of carbon occurs in this reaction?
A) sp to sp2
B) sp2 to sp3
C) sp3 to sp
D) sp2 to sp
E) sp3 to sp2
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of hybridized orbitals that can be formed by a fluorine atom?
A) 1
B) 2
C) 3
D) 4
E) 6
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the sulfur atom in SCl2?
A) sp
B) sp2
C) sp3
D) none
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry around a central atom that is sp3 hybridized and has one lone pair of electrons?
A) bent
B) linear
C) trigonal-planar
D) trigonal-pyramidal
E) tetrahedral
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an atom in a molecule or ion is described as sp3 hybridized,its electron pair geometry is
A) linear
B) triangular planar
C) triangular pyramidal
D) bent
E) tetrahedral
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s) .For oxygen and fluorine,the 2p orbital should be lower in energy than the 2p orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. ![Diagram 9-1 The molecular orbital diagram below may be used for the following problem(s) .For oxygen and fluorine,the \sigma <sub>2p</sub> orbital should be lower in energy than the \pi <sub>2p</sub> orbitals.However,the diagram will still yield correct bond order and magnetic behavior for these molecules. -Refer to Diagram 9-1.What is the molecular orbital configuration of F<sub>2</sub>? A) [core electrons] ( \sigma <sub>2s</sub>) <sup>2</sup> ( \sigma *<sub>2s</sub>) <sup>2</sup> ( \pi <sub>2p</sub>) <sup>4</sup> ( \sigma <sub>2p</sub>) <sup>2</sup> ( \sigma *<sub>2p</sub>) <sup>2</sup> B) [core electrons] ( \sigma <sub>2s</sub>) <sup>2</sup> ( \sigma *<sub>2s</sub>) <sup>2</sup> ( \pi <sub>2p</sub>) <sup>2</sup> ( \sigma <sub>2p</sub>) <sup>2</sup> ( \sigma *<sub>2p</sub>) <sup>2</sup> C) [core electrons] ( \sigma <sub>2s</sub>) <sup>2</sup> ( \sigma *<sub>2s</sub>) <sup>2</sup> ( \pi <sub>2p</sub>) <sup>4</sup> ( \pi *<sub>2p</sub>) <sup>4</sup> D) [core electrons] ( \sigma <sub>2s</sub>) <sup>2</sup> ( \sigma *<sub>2s</sub>) <sup>2</sup> ( \pi <sub>2p</sub>) <sup>4</sup> ( \sigma <sub>2p</sub>) <sup>2</sup> ( \sigma *<sub>2p</sub>) <sup>6</sup> E) [core electrons] ( \sigma <sub>2s</sub>) <sup>2</sup> ( \sigma *<sub>2s</sub>) <sup>2</sup> ( \pi <sub>2p</sub>) <sup>4</sup> ( \sigma <sub>2p</sub>) <sup>2</sup> ( \pi *<sub>2p</sub>) <sup>4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ea8937_ab6c_c408_a16d_5b406ef1d09a_TB4499_00.jpg) -Refer to Diagram 9-1.What is the molecular orbital configuration of F2?
-Refer to Diagram 9-1.What is the molecular orbital configuration of F2?
A) [core electrons] ( 2s) 2 ( *2s) 2 ( 2p) 4 ( 2p) 2 ( *2p) 2
B) [core electrons] ( 2s) 2 ( *2s) 2 ( 2p) 2 ( 2p) 2 ( *2p) 2
C) [core electrons] ( 2s) 2 ( *2s) 2 ( 2p) 4 ( *2p) 4
D) [core electrons] ( 2s) 2 ( *2s) 2 ( 2p) 4 ( 2p) 2 ( *2p) 6
E) [core electrons] ( 2s) 2 ( *2s) 2 ( 2p) 4 ( 2p) 2 ( *2p) 4
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 72
Related Exams