A) H2S
B) SO2
C) SF6
D) MgSO4
E) H2SO4
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formal charge on oxygen in the following structure? 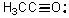
A) +2
B) +1
C) 0
D) -1
E) -2
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
An orbital is defined as a region of space where the probability of ___ is high.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
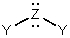 is a generalized structural representation which can be used for all of the following,except:
is a generalized structural representation which can be used for all of the following,except:
A) H2O
B) H2Se
C) H2S
D) BeH2
E) There is no exception.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Constitutional isomers differ in the ___.
Correct Answer

verified
connectivi...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which structure(s) contain(s) an oxygen that bears a formal charge of +1? 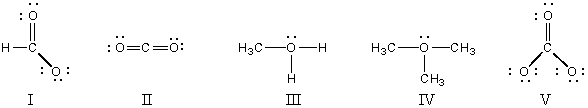
A) I and II
B) III and IV
C) V
D) II
E) I and V
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Essay
Determine the bond angles (I - IV)on the following structure based according to the VSEPR theory. 
Correct Answer

verified
I = 109.5o,...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Identify the atomic orbital the lone pair electrons on the O atom are contained in: 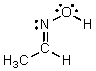
A) 2sp2
B) 2sp3
C) 2sp
D) 2s
E) 2p
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the 1s orbitals of two hydrogen atoms combine to form a hydrogen molecule,how are the electrons distributed in the resulting molecular orbitals?
A) Two electrons in the bonding molecular orbital.
B) One electron in the bonding molecular orbital,one electron in the non-bonding molecular orbital.
C) One electron in the bonding molecular orbital,one electron in the antibonding molecular orbital.
D) Two electrons in the non-bonding molecular orbital.
E) Two electrons in the antibonding molecular orbital.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule would be linear? (In each case you should write a Lewis structure before deciding.)
A) SO2
B) HCN
C) H2O2
D) H2S
E) OF2
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the spatial arrangement (shape) of the atoms of the methyl anion :CH3-?
A) octahedral
B) tetrahedral
C) trigonal planar
D) linear
E) trigonal pyramidal
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
When atomic orbitals of opposite phase overlap a(n)___ molecular orbital is formed.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a set of constitutional isomers? 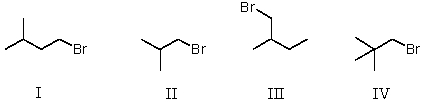
A) I and II
B) II and III
C) I,II,and III
D) II,III,and IV
E) I,III,and IV
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to molecular orbital theory,which molecule could not exist?
A) H2
B) He2
C) Li2
D) F2
E) N2
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the atomic orbitals in the N-O sigma bond in the following oxime: 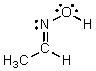
A) (2sp2,2sp2)
B) (2sp3,2sp3)
C) (2sp,2sp)
D) (2sp2,2sp3)
E) (2sp,1s)
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons contribute to pi bonds in the following compound? 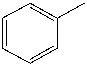
A) 0
B) 3
C) 6
D) 7
E) None of these choices.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the most electronegative element from the list below.
A) H
B) O
C) N
D) B
E) C
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The bond angle for the C-C-H bonds in CH3CN would be expected to be approximately:
A) 90
B) 109
C) 120
D) 145
E) 180
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 141 - 158 of 158
Related Exams