B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the ideal bond angles for H2O?
A) 120°
B) 109.5°
C) 90°
D) 180°
E) 75°
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Molecular orbitals are formed by adding and subtracting atomic orbitals.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the Xe atom in XeF2?
A) sp3d
B) sp
C) sp3
D) sp2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The three molecular shapes an sp3 hybridized molecule can have are ________.
A) triangular, bent, T-shaped
B) irregular tetrahedron, T-shaped, linear
C) octahedron, square pyramid, square planar
D) tetrahedron, trigonal pyramid, bent
E) tetrahedron, T-shaped, bent
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the INCORRECT statement about AsF6-.
A) The hybridization is sp3d2.
B) The molecule is octahedral.
C) There are no π bonds.
D) There is one lone pair of electrons.
E) There are six σ bonds.
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The octahedral hybrid configuration is composed of which orbital combination?
A) sp3d2
B) sp2d3
C) spd2
D) sp3d
E) sp3
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is INCORRECT?
A) Delocalized pi orbitals are formed when electrons are shared by unhybridized p orbitals in more than two atoms.
B) The band theory is a form of molecular orbital theory.
C) In band theory, there are two bands.
D) The valence band is at higher energy than the conduction band.
E) An energy band partially filled with electrons is a conduction band.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to principles of VSEPR theory applied on AsCl52-, which of the following is INCORRECT?
A) VSEPR formula = AX5E
B) molecular geometry = square planar
C) electron pair geometry = octahedral
D) hybridization = sp3d2
E) one lone pair and 5 bonding pairs
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the molecule 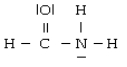
A) the hybridization for C is sp3
B) the hybridization for N is sp2
C) the hybridization for O is sp3
D) N is not hybridized
E) the hybridization for C is sp2
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the band theory of bonding, the smallest energy difference between a conduction band and a valence band typically occurs in substances such as:
A) crystalline rock salt
B) crystalline rubidium
C) crystalline silicon
D) crystalline iodine
E) crystalline arsenic
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The valence-bond method provides energy information about molecules.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which hybrid orbitals are impossible in electrically neutral molecules containing only the elements shown?
A) sp3/Al and H
B) sp2/B and F
C) sp/C and H
D) sp2/N and O
E) sp3/O and F
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the INCORRECT statement about H3O+.
A) There is one π bond.
B) There are 3 σ bonds.
C) There is one lone pair on O.
D) The hybridization on O is sp3.
E) The OH bonds are O(sp3) - H(1s) .
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the pairs of molecules below have the same hybridization on the central atom? (The central atom is underlined in each molecule.)
A) CO2, CH4
B) H2CO, BeH2
C) BCl3, HNO
D) H2O, HF
E) NH3, HNO
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A junction in a photovoltaic (solar) cell is which of the following?
A) a thin layer of n-type semiconductor in contact with a p-type semiconductor
B) three pieces of semiconductor that form a p-n-p connection
C) wires that carry electricity that are attached to the cell
D) pure germanium that is attached to a p-type semiconductor
E) a thin layer of p-type semiconductor in contact with an n-type semiconductor
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to MO theory, all of the following have a bond order of 2 EXCEPT:
A) NO
B) CN+
C) C2
D) O2
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about extrinsic semiconductors is INCORRECT?
A) They are generally made from pure Si or Ge with small amounts of impurities.
B) n-type are those in which the impurity is an element of Group 15.
C) p-type are those in which the impurity is an element of Group 13.
D) The crystal need not be very pure before the impurity is added to produce the semiconductor.
E) Boron is a common impurity in n-type extrinsic semiconductors.
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the hybridization of the N in the benzene-like molecule (C5H5N) ?
A) sp
B) sp3
C) sp3d2
D) sp3d
E) sp2
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is correct for the structure shown? 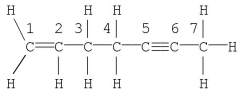
A) Carbon no. 1 is described by sp3 hybridization.
B) The molecule contains 19 σ bonds.
C) Carbon no. 2 is described by sp2 hybridization.
D) The molecule contains a total of five π bonds.
E) Carbon no. 7 is described by sp hybridization.
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 97
Related Exams